Introduction – Time For Ice Cubes To Freeze
The short answer is 2-4 hours to be completely frozen. Based on scientific experiments, the initiation of freezing varies between 30 minutes to 1 hour and 40 minutes depending on the initial temperature of the water.
There are controlled experiments carried out by many researchers that are published to confirm the times. But read on if you want to know how to make ice cubes freeze faster. Here at Shrink that Footprint we talk a lot about energy, heating and cooling. Today we’re going to talk about a practical topic. It’s a question that has been asked by many people over the years. How long for ice cubes to freeze? The answer, of course, depends on several factors, such as the ambient temperature and how much water is in the ice cube.
It is a hot summer day and you are out in the sun, working up a sweat. You head inside to get a drink and there, in your glass, are two ice cubes, slowly melting. You take a sip of your drink and feel the refreshing coldness of the ice cubes, cooling you down from the inside out. Ice cubes in drinks are not just a matter of taste – they can also have a significant impact on your health.

In hot weather, our bodies lose fluids through sweating, and it is important to replace these fluids to avoid dehydration. Drinking cold water or other fluids helps to cool our bodies down and prevents us from overheating. Ice cubes can also help to numb a sore throat or relieve pain from teething in infants.
In this blog post, we will explore some things to know about how long to make ice cubes for your drinks.
How Long For Ice Cubes To Freeze?
It takes ice cubes about two to four hours to freeze in a standard home freezer. However, several factors can affect this time, including the size of the ice cubes and the ambient temperature. In general, larger ice cubes will take longer to freeze than smaller ones. Likewise, if the temperature outside is warm, it will take longer for the ice cubes to freeze than if it is cold.
How Long For Metal Ice Cubes To Freeze?
“Metal ice cubes” are actually small metal cubes that enclose a material. It takes a few hours, like water ice cubes, for metal ice cubes to freeze. Metal conducts heat well, so the heat from the water around the metal cubes is quickly transferred to the interior itself.
However, there are a few factors that can affect how long it takes for metal ice cubes to freeze. For example, if the metal is in contact with air, it will take longer to freeze because air is a poor conductor of heat. In addition, if the metal is not in contact with water, it will take longer to freeze because there needs to be a source of heat to transfer to the metal. as long as the metal is in contact with both water and air, it will usually freeze within minutes.
How Does Temperature Impacts Ice Cube Freezing Time?

When ambient temperature decreases, the rate of “cold” transfer from the surroundings to the ice cubes increases. The increased rate of heat transfer causes the ice cubes to freeze faster. Conversely, when ambient temperature increases, the rate of “cold” transfer from the surroundings to the ice cubes decreases. This decrease in heat transfer causes the ice cubes to take longer to freeze.
The amount by which temperature affects the freezing time of ice cubes is dependent on the type of material from which the ice cube tray is made. Ice cube trays made from materials with high thermal conductivity, such as metal ice cubes or stainless steel ice cubes, allow cold to flow more readily from the warm surroundings into the ice cube tray. As a result, these trays cause the ice cubes to freeze faster than trays made from materials with low thermal conductivity, such as plastic.
In addition, insulation can also affect how long it takes for ice cubes to freeze. For example, if an ice cube tray is placed inside a foam cooler, it will take longer for the ice cubes to freeze than if the tray were placed in a room with no insulation. This is because foam coolers slow down the flow of heat, preventing it from easily reaching the ice cube tray. As a result, it takes longer for the ambient temperature to lower enough to cause the water in the tray to freeze. Ultimately, both material and insulation affect how long it takes for ice cubes to freeze.
Could Hot Water Freeze Faster Than Cold Water – Using The Mpemba Effect
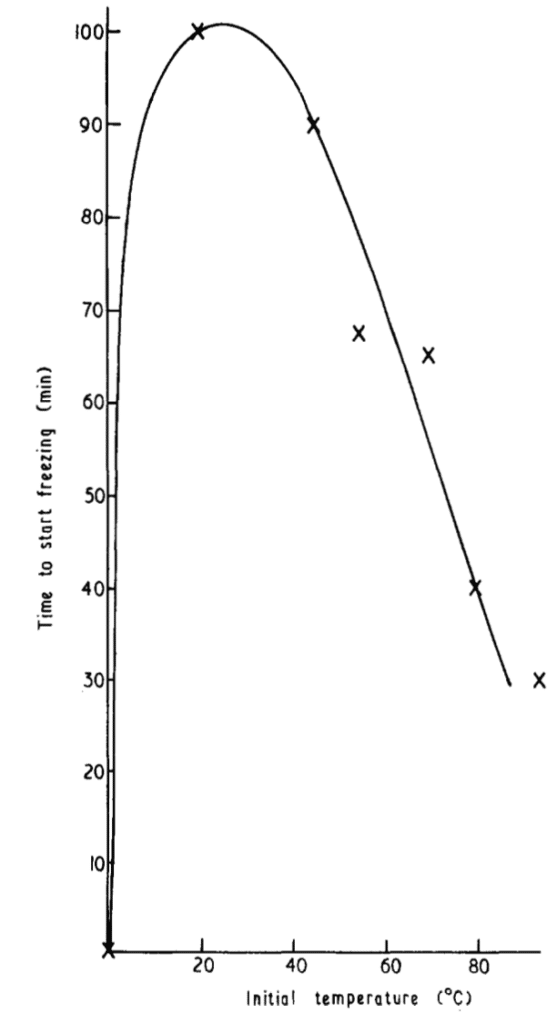
We keep talking about how lower freezer temperatures means faster freezing. But we haven’t talked yet about the initial temperature of the water. And it turns out to be quite complicated.
Hot water freezing faster than cold water is referred to as the Mpemba effect. This phenomenon is named after the Tanzanian student, Erasto Mpemba, who first observed it in the 1960s. He and his colleague published the observations in the scientific journal (Mpemba and Osborne 1969, IOP Science Physics Education).
The effect has been observed in many different liquids, including water, and has been studied by scientists for decades. The exact cause of this effect is still not fully understood, however, some theories suggest that it is due to the presence of dissolved gases, the beginning temperature of the water, or the rate of evaporation. The effect of hot water freezing faster than cold water is that it can reduce the amount of time and energy needed to freeze a given amount of liquid.
The experiments showed that very cold water indeed froze quickly. For example if chilled to near zero celsius, then it’d be a matter of 10-30 minutes before the water started to freeze. However, if the water started off at 20 degrees Celsius, which is room temperature, then it took 1 hour and 40 minutes to start freezing. This is in line with our estimates of 2-4 hours of ice cubes forming. But paradoxically, Mpemba and his colleagues found that as the initial temperature of the water increased, the freezing time curved back down again. Basically hotter water was getting to a freezing state faster!
Therefore, one of our recommendations is that if you want faster freezing of ice cubes, you should either use our rapid pre-chill method below, or a slightly warmer starting temperature!
Controversy Around The Mpemba Effect
However, we need to approach this subject with a degree of caution. A study published in Scientific Reports by Henry C. Burridge & Paul F. Linden titled “Questioning the Mpemba effect: hot water does not cool more quickly than cold” contradicts this phenomenon’s validity. This research was conducted in carefully controlled conditions, and the conclusion was quite disappointing for those who believed in the Mpemba effect: there’s no substantial evidence supporting the phenomenon.
After careful review and experimentation, the authors found no convincing evidence that hot water cools and subsequently freezes faster than cold water. This conclusion stands in stark contrast to the many anecdotes and some studies asserting the contrary.
How Much Water Impacts Ice Cube Freezing Time?
The freezing time of ice cubes is directly proportional to the water content of the cube. An ice cube with more water in it will take much longer to freeze. As a result of water’s high heat capacity, it can absorb a large amount of heat before becoming warm. Putting an ice cube in the freezer keeps it from freezing because the ice cube’s water is absorbed by that freezer’s heat. You should use less water if you want your ice cubes to freeze faster.

Tips To Help Speed Up The Process Of Freezing Ice Cubes
If you’re looking for ways to speed up the process of freezing ice cubes, there are a few things you can do. Here are some tips to help you out:
1. Fill your ice cube tray with cold water. This will help the water freeze faster.
2. Put the ice cube tray in the freezer close to the bottom because that’s where its coldest.
3. Use distilled water instead of tap water. The impurities of tap water reduce the freezing point. Impurities interfere with the regular lattice of ice forming.
4. Fill the tray half-way or part-way. The higher surface-to-volume ratio means heat will escape faster. In fact, if you decrease the water volume by half, the freezing will take place 4 times faster.
5. Don’t open the freezer door too often. Every time you open it, some of the cold air escapes, which can make the ice cubes take longer to freeze.
6. If possible, use a chest freezer instead of an upright freezer. Chest freezers maintain a more consistent temperature.
Extra Tip – Rapidly Chill Water Before Freezing
There is a way to chill the water rapidly before filling the ice cube maker tray. That is to use a chilling device. This is like tip #1 above but taking it to a more extreme degree. Amazon sells a number of these. They rely on a prefrozen container of water. Inside the container is a very high surface area space.
If you fill regular temperature water into this special container, the water rapidly chills. Pour this water into the ice cube maker tray. This will cut down on the cooling time needed to freeze the ice cubes. The product is called the “HyperChiller” and is basically a block of ice encased inside a core metal container with a very thin container volume wrapped around it that gives a large contact area between the ice and your liquid. Chilling to near zero occurs within minutes.

Conclusion
So there you have it, everything you need to know about freezing ice cubes. Now go out and experiment with different techniques to find the one that works best for you. And don’t forget to have fun in the process!
